Abstract
Background: Patients with lower-risk myelodysplastic syndromes (MDS) who develop red blood cells (RBC) transfusion-dependent anemia, experience worse quality of life and shorter survival. Therapeutic strategies after failure of erythropoiesis stimulating agents (ESAs) are limited. Regulatory approval of luspatercept in lower-risk MDS with ring sideroblasts (MDS-RS) offers a novel approach for the treatment of transfusion-dependent anemia in this subset of patients. However, data about luspatercept effectiveness in a real-world clinical setting are lacking. Here we report the results of a multicenter retrospective study including patients who were treated with luspatercept within the Italian compassionate use program.
Methods: Eligible patients were ≥ 18 years old, had MDS-RS (2016 WHO classification), met criteria for very low, low, or intermediate IPSS-R risk; were receiving regular RBC transfusions (≥2 units/8 weeks in the 16 weeks before study entry); were refractory to or unlikely to respond to ESAs. Primary end point was transfusion independence (TI) for 8 weeks or longer during weeks 1 through 24; key secondary end point was TI for 12 weeks or longer, assessed during both weeks 1 through 24 and weeks 1 through 48. Other secondary end points included erythroid response (defined by the IWG 2006 criteria), progression to acute myeloid leukemia (AML) and safety analyses. Exploratory analyses were performed to identify predictive factors for treatment response.
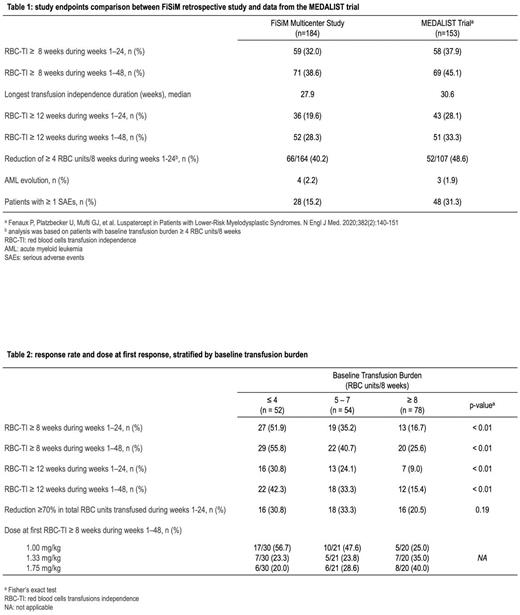
Results: 184 patients who were enrolled in the Italian luspatercept compassionate use program between December 2020 and December 2021 were analyzed in this study. Median age at enrollment was 74 years (range, 31-88), median time since diagnosis was 43 months, and median time since onset of transfusion-dependence was 23 months. Overall, 123 (66.8%) patients had at least one comorbidity requiring ongoing treatment, 39 (21.2%) had at least three. Baseline median transfusion burden was 6 RBC units every 8 weeks (range, 2-25). At the time of data analysis (June 30, 2022), 130 (73.4%) patients completed at least 24 weeks of treatment. Median time on treatment was 301.5 days, and median follow-up time was 376 days. During the first 24 weeks of the trial, 59 (32%) patients had TI for 8 weeks or longer. The percentage of patients achieving TI increased to 38.6% in the first 48 weeks of the trial. Median duration of TI was 27.9 weeks. Focusing on secondary endpoints, 36 patients (19.6%) had TI for 12 weeks or longer during the first 24 weeks, 52 (28.3%) had TI for 12 weeks or longer considering the first 48 weeks of the trial. A reduction ≥ 4 RBC units/8 weeks during weeks 1-24 was seen in 66 patients (40.2%). Four (2.2%) patients evolved to AML. Serious adverse events were recorded in 27 (15.2%) patients and led to study discontinuation in 5 (2.8%). Overall, treatment was discontinued in 72 (40.7%) patients, mainly for lack of benefit (table 1) The maximum approved dose (1.75 mg/kg) of luspatercept was administered at least once in 144 (81%) patients. Among patients who achieved TI for 8 weeks or longer in the first 48 weeks, luspatercept dose (mg/kg) at response was 1, 1.33 and 1.75 in 31 (46.3%), 18 (29.9%) and 18 (29.9%%) patients, respectively. Logistic regression analysis showed that higher baseline transfusion burden was significantly associated with a lower probability of achieving TI. We identified 3 group of patients stratified according to baseline transfusion burden (group 1, ≤4 units/8 weeks; group 2, 5 to 7 units/8 weeks, group 3, ≥8 units/8 weeks) with a significantly different probability to achieve both the primary endpoint (51.9%, 35.2% and 16.7%, respectively, P<0.01) and the key secondary endpoint (30.8%, 24.1% and 9%, respectively, P<0.01). Patients with higher transfusion burden had higher luspatercept doses at first response (table 2). In patients with low probability to achieve TI (group 2 and 3), a reduction in transfusions equal or greater than 4 units/8 weeks was achieved in 66 (46.2%).
Conclusion: Luspatercept was effective for the treatment of transfusion-dependent anemia in MDS-RS in a real-life setting. The benefit extended beyond the achievement of TI and produced a significant reduction in the number of RBC transfusions. Higher baseline transfusion burden was associated with a reduced probability to achieve TI; in these patients, the reduction of transfused RBC units appears a more reliable treatment target.
Disclosures
Fattizzo:Sobi: Speakers Bureau; Alexion: Consultancy, Speakers Bureau; Amgen: Consultancy; Janssen: Consultancy; Momenta: Consultancy; Novartis: Consultancy, Speakers Bureau. Oliva:BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Patents & Royalties: HM PRO, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Sobi: Honoraria; Daiichi: Consultancy; Janssen: Consultancy; Apellis: Consultancy, Honoraria. Voso:Astellas: Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; jazz: Consultancy, Speakers Bureau. Elena:Gilead: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Pane:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte, BMS, Janssen, Amgen, Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Speakers Bureau. Breccia:Novartis, Incyte, Pfizer, BMS, Abbvie: Honoraria. Galimberti:Abbvie Incyte, Novartis, Janssen, Astrazeneca, Pfizer: Honoraria. Rambaldi:Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Incyte: Honoraria; Roche: Honoraria; Janssen: Honoraria; Omeros: Honoraria; Celgene-BMS: Honoraria; Pfizer: Honoraria; Amgen: Honoraria. Santini:Novartis: Honoraria; AbbVie: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Srvier: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal